Molecular Dynameomics
Key Challenges: Perform molecular dynamics simulations to characterize both native (i.e. biologically active) and high-temperature state and folding / unfolding pathways representing all 1,695 known protein fold families. Carry out a large-scale assessment of nativeprotein flexibility by examining a database containing the simulation results. Extract information about dominant dynamical modes and significant information about potential conformations available to important proteins. Dynameomics is the name of the project carrying out this work as well as the name of the resulting database.
Why it Matters: Structure prediction remains one of the elusive goals of protein science. This work will provide a broad knowledgebase of structural pathways to support research in bioenergy production, environmental remediation, and carbon cycling. Misfolding of proteins is also implicated in a large number of diseases. Because proteins are not static entities (as they are represented in structural databases) and because crystal structures do not necessarily represent a protein in its active conformation, flexibility data are critical in determining protein behavior and function.
Accomplishments: Over 11,000 protein folding simulations are now available in the world’s largest public database of protein structures. These simulations have revealed a common structural feature in the development of amyloids, toxic protein clusters that are implicated in more than two dozen diseases. A compound designed to bind with this structure has been shown to inhibit amyloid formation in three types of proteins, suggesting a possible strategy for drug design.
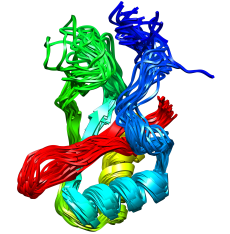
Among the many important accomplishments from this work is an explanation for the stability of thermophilic proteins. (These are proteins from organisms that t hrive at relatively high temperatures - up to 176 °F). The simulations identified a network of electrostatic interactions within a specific region of the protein (shown at right) that appears to allow the body of the protein to retain its native conformation to a much greater degree at high temperature.
hrive at relatively high temperatures - up to 176 °F). The simulations identified a network of electrostatic interactions within a specific region of the protein (shown at right) that appears to allow the body of the protein to retain its native conformation to a much greater degree at high temperature.
Investigators: Valerie Daggett research group, University of Washington
More Information: See Protein Engineering Design & Selection, 23:327-336, 2010, and depts.washington.edu/daglab/