EFRC Carbon Capture and Sequestration Activities at NERSC
Why it Matters: Carbon dioxide (CO2) gas is considered to be present in only trace proportions in our atmosphere but it has a leading role in the cast of greenhouse gases, with a thermal radiative effect nearly three times as large as the next biggest contributor. Energy related processes are the biggest sources of atmospheric CO2, especially the burning of fossil fuels and the production of hydrogen from methane. Since both human-caused CO2 concentrations and global average temperatures have been increasing steadily since the mid-20th century it could very well be that our energy future depends on our ability to effectively remove CO2 from the atmosphere. But there are significant technological challenges.
Several NERSC projects associated with Energy Frontier Research Centers (EFRCs) are seeking to surmount those challenges by simulating some of the complicated processes related to underground carbon capture and sequestration. These are geoengineering techniques that remove greenhouse gases from the atmosphere and prevent them from re-entering. Two example projects are described below.
Project: Computational Characterization of Porous Materials at the Center for Gas Separations Relevant to Clean Energy Technologies EFRC
Objective: The vision of this EFRC is development of new methods to understand, predict, and create novel materials with optimal molecular properties for carbon capture and sequestration. Separation of CO2 from other gases is a key technological bottleneck. Nanoporous materials are attractive choices for gas separation and are already widely used in the chemical industry. Zeolites, complicated microporous aluminosilicate minerals, are a good example. The number of possible zeolite structures has been estimated to be more than 2.5 million, although only about 190 structures have been synthesized to date. Complete characterization of an entire database of hypothetical structures would be out of the question even with today's supercomputers.
The specific goal, then, is to develop alternative approaches for screening large databases of these complex materials so that only those structures predicted to exhibit properties of interest are subjected to follow-on characterizations that use more accurate but also more expensive molecular simulation methodology. Finding the right material for separation of CO2 from gas mixtures will significantly reduce the costs associated with carbon capture.
Principal Investigators: B. Smit, M. Haranczyk, J. Sethian (LBNL and UC Berkeley);
Accomplishments: NERSC resources have already been used to develope an automated method to bypass a manual, time-consuming, visual analysis of void spaces in porous materials. This will enable high-throughput, unsupervised molecular simulations and it means that NERSC resources can now be used at a larger scale to characterize many more materials. This work is also related to the BES/ASCR SciDAC SAP project "Knowledge Guided Screening Tools for identification of Porous Materials for CO2 Separations."
More Information: See J. Chem. Theory Comput. 6, 3472–3480 (2010) and the Energy Frontier Research Center for gas separations relevant to clean air technologies web site.
Project: Clay Mineral Surface Geochemistry Studies for the Center for Nanoscale Control of Geologic CO2 EFRC
Objective: A portion of the research at this LBNL Energy Frontier Research Center seeks to investigate molecular-scale processes relevant to the sequestration of CO2 in geologic formations. The performance of CO2 repositories depends strongly on the ability of nanoporous clay seals to prevent CO2 escape and on the efficiency with which the reservoir pore space can be filled with CO2. Our need to predict and optimize the behavior of CO2 in geologic media depends on our ability to gain insight into mineral-water-CO2 interfacial phenomena via molecular-scale simulation.
Principal Investigators:
G. Sposito, I. Bourg (LBNL); L. Nielsen (UC Berkeley)
Accomplishments: Current investigations have focused on the structure of the electric double layer (EDL) on flat charged surfaces (smectite basal surfaces) in aqueous brines and on the CO2-brine interfacial tension and solubility properties under conditions relevant to CO2 sequestration. Molecular dynamics simulations have already yielded an unprecedentedly detailed view of water/ion distribution and dynamics near clay surfaces. Other studies will focus on molecular simulations of water, CO2, and brine near solid surfaces, especially the nanopore between two clay layers. They will seek to know if CO2 is absorbed at the water surface, how fast water and CO2 molecules transfer across the interface, does an electric double layer form at the interface and if so, would it affect water and CO2 structure and dynamics by making the fluid more viscous.
More Information: See Journal of Colloid and Interface Science 360 701–715 (2011), Environ. Sci. Technol. 44 2085–2091 (2010), and the Center for Nanoscale Control of Geologic CO2 web site.
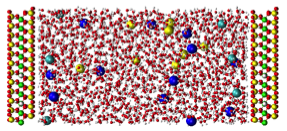
Snapshot of NERSC molecular dynamics simulation showing brine confined in a 58-Å wide nanopore between two smectite clay surfaces.







