Novel Methods for Harvesting Solar Energy
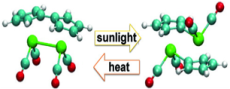
Model of a molecule that reversibly changes it structure when it absorbs light. Simulations at NERSC revealed the mechanism for the heat-releasing step (2->1) and suggest substantial promise for creating batteries from molecules that can reversibly store solar energy as heat.
Why it Matters: Solar thermal fuels, which store energy from the sun in chemical bonds, are a tantalizing energy storage prospect: in principle they are 100% renewable, produce no emissions, are easily transportable, and can be recharged without any special equipment.
Key Challenges: The challenge is reversibility. Only one material is known that can reversibly convert light to chemical energy and it is based on an expensive metal. Finding additional solar fuel candidates that can perform the process repeatedly with no degradation requires a novel first principles-based high-throughput screening method to search several thousand known photochromic molecules.
Accomplishments: Using first-principles quantum-mechanical calculations at NERSC, researchers are studying the properties of a molecule that can absorb photons and later release the stored energy as heat in the presence of a simple catalyst. The calculations revealed an unexpected second energy barrier in the reaction pathway that makes the charged state of the molecule extremely stable. Using this knowledge, researchers are working to make materials cheaper and more efficient. The work has led to a deeper understanding of the molecular and electronic properties that give rise to the desired photoactive behavior, and will help enable intelligent design of completely new molecules and molecular systems for solar energy storage applications.
Investigators: Jeffrey Grossman (MIT)
More Information: See, for example, Angewandte Chemie International Edition, Vol. 49, Issue 47, pages 8926–8929, November 15, 2010, and Professor Grossman's web site.







