Chemistry of Metal Contaminants in Water
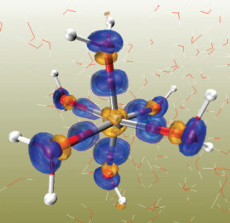
A plot of difference electron density of a Zn2++6H2O/250H2O system from AIMD/MM calculations, minus the Zn2+ and water components, i.e., Zn2+ /250H2O and 6H2O/250H2O. The surplus charge distribution is shown in the transparent blue isosurfaces, and the deficit charge distribution is shown in the transparent orang isosurfaces.
Key Challenges: Molecular-level studies of highly-charged metal ions in aqueous solution to gain first-principles theoretical interpretation of solvation structure and dynamics for a variety of geochemically-relevant conditions. Modeling charged ions in solution is challenging because the water molecules surrounding the ion are strongly polarized. The goal is to determine structure of regions beyond the first hydration shell and relate the structure to the substantial differences in chemical properties that exist for these ions.
Why it Matters: Metals such as zinc and iron are essential nutrients for living organisms and their biological role is fairly well understood but much of the chemistry of their ions in solution has not been explained from first principles. Detailed understanding of solvation processes (those occurring when heavy metals dissolve in water) is crucial for understanding the transport of toxic materials. The detailed structure of the hydration region surrounding an ion is difficult to observe experimentally placing more importance on accurate simulation studies. For elements such as uranium solvation structure is important for predicting the long-term viability of proposed containment strategies, since the most likely way for uranium to migrate into the biosphere is through groundwater coming in contact with containment canisters resulting in solvated complexes.
Accomplishments: Separate studies of iron, zinc, and uranium have been published using NERSC as well as other computational facilities. A system with one iron ion and 64 H2O molecules (517 electrons) was simulated for 30 picoseconds, which was five times longer and involved two times more H2O’s than had been done previously. This study clearly demonstrated the importance of the first-principles approach afforded by the use of extreme scale computing. Two different NWChem ab initio-DFT analyses of the uranium oxide ion (UO22+), one with 64 water molecules for 22 ps and one with 122 waters for 9 ps were also carried out. These were extremely-demanding simulations due to large number of water molecules and long integration times. The results helped explain X-ray spectra but also revealed additional structural features. Finally, both ab initio and ab initio/classical molecular dynamics simulations of the Zn2+ ion in aqueous solution using 64 and 256 water molecules was also carried out. The structure of the second hydration shell disagrees with results reported from conventional molecular dynamics simulations.
The PNNL researchers involved in this work collaborate with NERSC on porting and optimizing the NWChem suite of computational chemistry tools so that it is available for all users on NERSC systems.
Investigators: Stuart Bogatko, Eric Bylaska, and Andrew Felmy (PNNL); John H. Weare (University of California San Diego)
More Information: See the Journal of Chemical Physics 128 124507 (2008); J. Phys. Chem. A 114 2189–2200 (2010); and Journal of Chemical Physics 132, 194502 (2010)







